Abstract
The bone marrow (BM) microenvironment regulates acute myeloid leukemia (AML) initiation, proliferation and chemotherapy resistance. Following cancer cell death, a growing body of evidence suggests an important role for uncleared apoptotic debris in regulating the immunologic response to, and growth of, solid tumors. LC3-associated phagocytosis (LAP) maintains tissue homeostasis by regulating immune responses, such as tumor immunity. Here we investigate the role of LAP in macrophage within the BM microenvironment of AML.
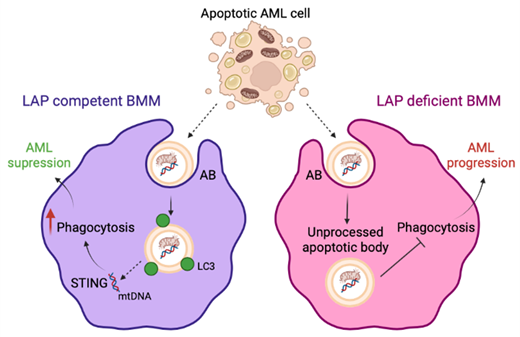
We find that depletion of BM macrophages via clodronate liposomes increased AML growth in-vivo. We show that LAP is an important pathway in BM macrophage to process dead and dying cells in the AML microenvironment. We used two syngeneic leukemia models (HOXA9/Meis1 and MN1) to investigate the role of LAP on AML proliferation. AML cells were injected into LAP deficient (Atg16L1 E230-) and wild-type (Atg16L1 E230+) mice. Targeted inhibition of LAP leads to accumulation of apoptotic cells (AC) and apoptotic bodies (AB) in the tumor microenvironment resulting in accelerated leukemia growth and decreased animal survival.
Mechanistically, we show, via cytokine arrays and gene analysis, that the phagocytosis of AML derived AB via LAP in BM macrophage resulted in STING pathway activation in the phagocytic cells. Furthermore, through inhibition of STING using H-151 STING inhibitor, we show that STING activation in vivo supressed leukemia growth. STING activation can lead to a type I IFN response and to recruitment of cytotoxic T-cells. We saw no increase in CD8 + T-cell numbers or activation, however, via ex vivo analysis found that STING activation is required for phagocytic functions in macrophages.
Next, we found that leukemic AB can induce a STING response in BM derived macrophages and that leukemic AB have increased mitochondria content that are processed by macrophages. Moreover, we identify that mitochondrial damage associated molecular patterns (DAMPs) from leukemic AB are processed by BM macrophages via LAP. Additionally, the depletion of mitochondrial DNA (mtDNA) in AML derived AB identified that the mtDNA from leukemic AB is responsible for the induction of STING signalling in BM macrophages.
In summary, we report that LAP in BM macrophage of apoptotic debris in the AML microenvironment suppresses leukemic growth, through mechanisms stimulated by AML apoptotic bodies which contain mtDNA in the BM microenvironment. This process is mediated by the activation of the STING pathway.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal